Treatment of Newly Diagnosed Myeloma - Transplant Eligible
Poster Session 1
P-158: EXPERIENCE AT SON ESPASES UNIVERSITARY HOSPITAL WITH DARATUMUMAB, BORTEZOMIB, LENALIDOMIDE AND DEXAMETHASONE (D-VRd) AS FIRST LINE TREATMENT IN MULTIPLE MYELOMA
Wednesday, September 27, 2023
1:30 PM - 2:30 PM EEST

Lola Piquer Monsonis (she/her/hers)
Resident
Hospital Universitari Son Espases, United States
Introduction: Multiple myeloma (MM) is characterized by uncontrolled plasma cell proliferation, anaemia, renal failure, hypercalcemia, osteolytic bone lesions, and immunodeficiency.
First line treatment in patients who are candidates for autologous stem cell transplantation (ASCT) is based on triplets, basically the combination of bortezomib, lenalidomide and dexamethasone (VRd) during 4-6 cycles. The latest studies add a 4th drug to current triplets, a monoclonal antibody against CD38 which seems to improve treatment responses without increasing toxicity. The main aim of our research is to show our experience on a third level hospital with a quadruple induction regimen (D-VRd) as first line treatment in MM.
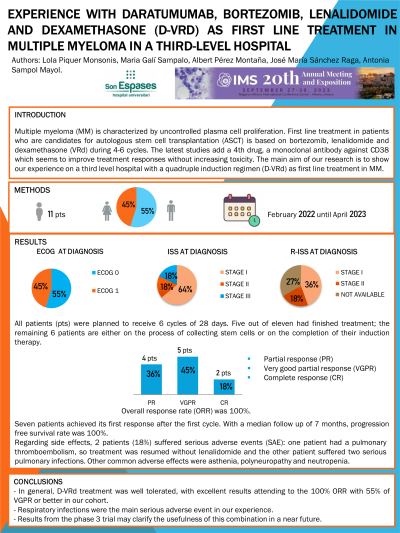
Methods: A retrospective descriptive study was carried out analyzing 11 patients diagnosed with MM and treated in first line with D-VRd from February 2022 until April 2023 at Hospital Universitari Son Espases. We analyzed clinical-biological parameters, prognostic staging systems, treatment, and clinical outcomes.
Results: We analyzed 11 patients, 6 of whom were male (55%). Median age was 57 years old (range 41-69). Six patients had performance status ECOG 0 and 5 patients ECOG 1 at diagnosis. Based on the International Staging System (ISS): 7 patients (64%) were stage I, 2 (18%) stage II and 2 (18%) stage III. On the other hand, when analyzing R-ISS: 4 patients (36%) were stage I, 2 (18%) stage II, 2 patients (18%) stage III and lastly 3 (27%) were not classified due to lack of FISH results. Regarding stratification risk based on FISH (R-ISS criteria): 2 patients were high risk; 6 were standard-risk and 3 unknown due to lack of FISH.
Average filtration rate at the beginning of treatment was 84 ml/min/1,73 m2 (range 21-104).
All patients were planned to receive 6 cycles of 28 days (bortezomib 1.3 mg/m2 on days 1,4,8,11; lenalidomide 25 mg for 21 days; dexamethasone 40 mg on days 1-4, 9-12). Five out of eleven had finished treatment; the remaining 6 patients are either on the process of collecting stem cells or on the completion of their induction therapy. Overall response rate (ORR) was 100%. Two patients (18%) achieved complete response (CR), 5 (45%) very good partial response (VGPR) and 4 (37%) partial response (PR). Seven patients achieved its first response after the first cycle. With a median follow up of 7 months, progression free survival rate was 100%.
Regarding side effects, 2 patients (18%) suffered serious adverse events (SAE): one patient had a pulmonary thromboembolism, so treatment was resumed without lenalidomide and the other patient suffered two serious pulmonary infections. Other common adverse effects were asthenia, polyneuropathy and neutropenia.
Conclusions: In general, D-VRd treatment was well tolerated, with excellent results attending to the 100% ORR with 55% of VGPR or better in our cohort. Respiratory infections were the main serious adverse event in our experience. Results from the phase 3 trial may clarify the usefulness of this combination in a near future.
First line treatment in patients who are candidates for autologous stem cell transplantation (ASCT) is based on triplets, basically the combination of bortezomib, lenalidomide and dexamethasone (VRd) during 4-6 cycles. The latest studies add a 4th drug to current triplets, a monoclonal antibody against CD38 which seems to improve treatment responses without increasing toxicity. The main aim of our research is to show our experience on a third level hospital with a quadruple induction regimen (D-VRd) as first line treatment in MM.
Methods: A retrospective descriptive study was carried out analyzing 11 patients diagnosed with MM and treated in first line with D-VRd from February 2022 until April 2023 at Hospital Universitari Son Espases. We analyzed clinical-biological parameters, prognostic staging systems, treatment, and clinical outcomes.
Results: We analyzed 11 patients, 6 of whom were male (55%). Median age was 57 years old (range 41-69). Six patients had performance status ECOG 0 and 5 patients ECOG 1 at diagnosis. Based on the International Staging System (ISS): 7 patients (64%) were stage I, 2 (18%) stage II and 2 (18%) stage III. On the other hand, when analyzing R-ISS: 4 patients (36%) were stage I, 2 (18%) stage II, 2 patients (18%) stage III and lastly 3 (27%) were not classified due to lack of FISH results. Regarding stratification risk based on FISH (R-ISS criteria): 2 patients were high risk; 6 were standard-risk and 3 unknown due to lack of FISH.
Average filtration rate at the beginning of treatment was 84 ml/min/1,73 m2 (range 21-104).
All patients were planned to receive 6 cycles of 28 days (bortezomib 1.3 mg/m2 on days 1,4,8,11; lenalidomide 25 mg for 21 days; dexamethasone 40 mg on days 1-4, 9-12). Five out of eleven had finished treatment; the remaining 6 patients are either on the process of collecting stem cells or on the completion of their induction therapy. Overall response rate (ORR) was 100%. Two patients (18%) achieved complete response (CR), 5 (45%) very good partial response (VGPR) and 4 (37%) partial response (PR). Seven patients achieved its first response after the first cycle. With a median follow up of 7 months, progression free survival rate was 100%.
Regarding side effects, 2 patients (18%) suffered serious adverse events (SAE): one patient had a pulmonary thromboembolism, so treatment was resumed without lenalidomide and the other patient suffered two serious pulmonary infections. Other common adverse effects were asthenia, polyneuropathy and neutropenia.
Conclusions: In general, D-VRd treatment was well tolerated, with excellent results attending to the 100% ORR with 55% of VGPR or better in our cohort. Respiratory infections were the main serious adverse event in our experience. Results from the phase 3 trial may clarify the usefulness of this combination in a near future.
