Cellular and T cell engager Immunotherapy
Poster Session 1
P-040: Idecabtagene vicleucel (ide-cel) versus standard regimens in patients with triple-class-exposed (TCE) relapsed and refractory multiple myeloma (RRMM): a KarMMa-3 high-risk subgroup analysis
Wednesday, September 27, 2023
1:30 PM - 2:30 PM EEST

Krina K. Patel, MD (she/her/hers)
Associate Professor
MD Anderson Cancer Center, University of Texas, Houston, TX, USA
Houston, Texas, United States
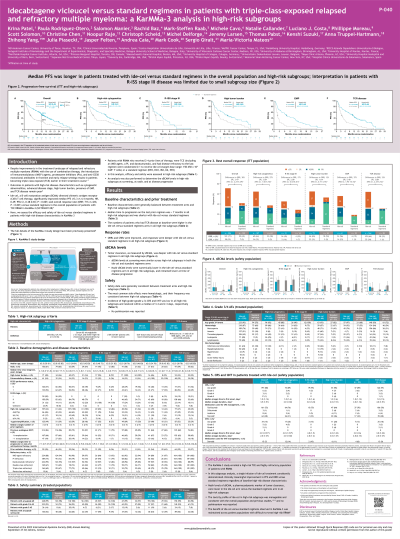
Introduction: Despite improvements in the treatment landscape of RRMM, outcomes remain poor in patients (pts) with high-risk features such as cytogenetic abnormalities, advanced-disease stage, high tumor burden (HTB), presence of extramedullary plasmacytoma (EMP), and triple-class refractory disease (TCRMM). Ide-cel, a BCMA-directed CAR T cell therapy, significantly improved median progression-free survival (mPFS; 13.3 vs 4.4 mo; HR 0.49; P< 0.001) and overall response rate (ORR; 71 vs 42%; P< 0.001) vs standard (std) regimens in the overall population of pts with TCE RRMM in KarMMa-3 (NCT03651128). Here we assess efficacy and safety of ide-cel vs std regimens in high-risk subgroups in KarMMa-3.
Methods: In KarMMa-3, pts with RRMM who received 2–4 prior regimens, who were TCE (immunomodulatory agent, proteasome inhibitor [PI], and daratumumab), and had disease refractory to the last regimen, were randomized 2:1 to receive ide-cel (target dose range: 150–450 x 10˄6 CAR+ T cells) or a std regimen (DPd, DVd, IRd, Kd, or EPd, based on prior regimen, per investigator). Efficacy was assessed in high-risk groups including pts with cytogenetic abnormalities (del[17p], t[4;14], or t[14;16]), R-ISS stage III disease, HTB (≥50% CD138+ plasma cells in bone marrow), EMP (soft-tissue-only and soft-tissue bone-related plasmacytomas), and TCRMM (refractory to ≥1 each of an IMiD® agent, a PI, and an anti-CD38 antibody).
Results: Baseline demographics and disease characteristics were balanced between arms. Median time to progression on the last prior regimen was short in pts treated with both ide-cel vs std regimens in all high-risk subgroups: cytogenetic abnormalities (5.6 vs 6.7 mo), R-ISS stage III disease (3.9 vs 3.5 mo), HTB (5.1 vs 6.2 mo), EMP (5.1 vs 5.1 mo), and TCRMM (5.6 vs 5.8 mo). At a median follow-up of 18.6 mo (range 0.4–35.4), mPFS was longer in pts treated with ide-cel vs std regimens in all high-risk subgroups: cytogenetic abnormalities (11.9 vs 4.2 mo; HR 0.608), R-ISS stage III disease (5.2 vs 3.0 mo; HR 0.861), HTB (11.0 vs 4.9 mo; HR 0.595), EMP (7.2 vs 2.0 mo; HR 0.401), and TCRMM (11.2 vs 3.5 mo; HR 0.458). ORRs (cytogenetic abnormalities [64.5 vs 37.7%], R-ISS stage III [45.2 vs 28.6%], HTB [64.8 vs 52.9%], EMP [55.7 vs 18.8%], and TCRMM [64.0 vs 31.5%]) and complete response rates (CRR; cytogenetic abnormalities [31.8 vs 4.9%], R-ISS stage III [16.1 vs 7.1%], HTB [31.0 vs 8.8%], EMP [23.0 vs 3.1%], and TCRMM [33.5 vs 1.1%]) were improved in pts treated with ide-cel vs std regimens in all high-risk subgroups. Safety data will be presented.
Conclusions: Pts treated with ide-cel had a lower risk of disease progression or death and higher odds of achieving an overall response (with higher CRRs) vs pts who received std regimens, regardless of baseline high-risk disease. These results support use of ide-cel in pts with TCE RRMM, including those with difficult-to-treat, high-risk disease.
Previously presented at European Hematology Association Meeting 2023, presentation S195.
Methods: In KarMMa-3, pts with RRMM who received 2–4 prior regimens, who were TCE (immunomodulatory agent, proteasome inhibitor [PI], and daratumumab), and had disease refractory to the last regimen, were randomized 2:1 to receive ide-cel (target dose range: 150–450 x 10˄6 CAR+ T cells) or a std regimen (DPd, DVd, IRd, Kd, or EPd, based on prior regimen, per investigator). Efficacy was assessed in high-risk groups including pts with cytogenetic abnormalities (del[17p], t[4;14], or t[14;16]), R-ISS stage III disease, HTB (≥50% CD138+ plasma cells in bone marrow), EMP (soft-tissue-only and soft-tissue bone-related plasmacytomas), and TCRMM (refractory to ≥1 each of an IMiD® agent, a PI, and an anti-CD38 antibody).
Results: Baseline demographics and disease characteristics were balanced between arms. Median time to progression on the last prior regimen was short in pts treated with both ide-cel vs std regimens in all high-risk subgroups: cytogenetic abnormalities (5.6 vs 6.7 mo), R-ISS stage III disease (3.9 vs 3.5 mo), HTB (5.1 vs 6.2 mo), EMP (5.1 vs 5.1 mo), and TCRMM (5.6 vs 5.8 mo). At a median follow-up of 18.6 mo (range 0.4–35.4), mPFS was longer in pts treated with ide-cel vs std regimens in all high-risk subgroups: cytogenetic abnormalities (11.9 vs 4.2 mo; HR 0.608), R-ISS stage III disease (5.2 vs 3.0 mo; HR 0.861), HTB (11.0 vs 4.9 mo; HR 0.595), EMP (7.2 vs 2.0 mo; HR 0.401), and TCRMM (11.2 vs 3.5 mo; HR 0.458). ORRs (cytogenetic abnormalities [64.5 vs 37.7%], R-ISS stage III [45.2 vs 28.6%], HTB [64.8 vs 52.9%], EMP [55.7 vs 18.8%], and TCRMM [64.0 vs 31.5%]) and complete response rates (CRR; cytogenetic abnormalities [31.8 vs 4.9%], R-ISS stage III [16.1 vs 7.1%], HTB [31.0 vs 8.8%], EMP [23.0 vs 3.1%], and TCRMM [33.5 vs 1.1%]) were improved in pts treated with ide-cel vs std regimens in all high-risk subgroups. Safety data will be presented.
Conclusions: Pts treated with ide-cel had a lower risk of disease progression or death and higher odds of achieving an overall response (with higher CRRs) vs pts who received std regimens, regardless of baseline high-risk disease. These results support use of ide-cel in pts with TCE RRMM, including those with difficult-to-treat, high-risk disease.
Previously presented at European Hematology Association Meeting 2023, presentation S195.
