Myeloma Genomics and cell signaling
Poster Session 3
P-339: Longitudinal Genetically Detectable Minimal Residual Disease by Interphase Fluorescence in Situ Hybridization Confers a Poor Prognosis in Multiple Myeloma
Friday, September 29, 2023
1:15 PM - 2:15 PM EEST
Jian Cui
postgraduate
Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Science & Peking Union Medical College
Tianjin, Tianjin, China (People's Republic)
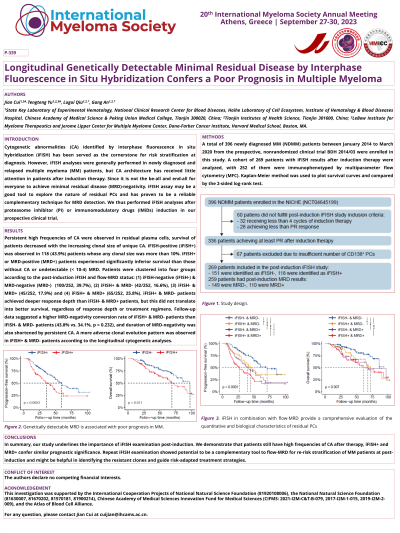
Introduction: Deeper depth of response (DpR) after induction therapy, especially gain of negative minimal residual disease (MRD), has been linked to prolonged survival in multiple myeloma (MM). However, flow-MRD examination focuses on the numbers, but not on the biological characteristics of residual plasma cells (PCs). To explore whether the genetic features of residual tumor cells affect the survival time of patients with MM, we investigated the clonality of cytogenetic abnormalities (CAs) of the residual PCs using fluorescence in situ hybridization (iFISH) in the National Longitudinal Cohort of Hematological Diseases in China (NCT04645199).
Methods: A total of 396 patients diagnosed with newly diagnosed MM (NDMM) between January 2014 and March 2020 were included in this study. EDTA-anticoagulated BM aspirate samples were collected at diagnosis for all 396 patients included in this study, and clonal PCs were enriched by CD138+ magnetic beads (Miltenyi Biotec, Paris, France). iFISH was then performed with probes specific for CAs as follows: 13q14, 17p13, 1q21, 14q32 (5’IGH, 3’IGH), t(4;14)(p16;q32), t(11;14)(q13;q32), and t(14;16)(q32;q23), and a total of 200 interphase nuclei were analyzed.
Results: Persistent CAs after induction therapy were detected in about half of the patients (118/269, 43%), and patients with undetectable CAs showed significantly improved survival compared with those with genetically detectable MRD (median progression-free survival [mPFS]: 59.7 months vs. 35.7 months, P < 0.001; median overall survival [mOS]: 97.1 months vs. 68.8 months, p = 0.011). Additionally, different patterns of therapy-induced clonal evolution were observed by comparing the clonal structure of residual PCs with paired baseline samples. Patients who maintained at high-risk during follow-up had the worst survival (mPFS: 23.0 months; mOS:40.4 months), while those returned to lower risk or had iFISH- at both time points had the best survival (mPFS: 59.7 months; mOS: 97.2 months).
Conclusions: These foundings highlighted the prognostic value of genetic testing in residual tumor cells after induction therapy, which may provide a deep understanding of clonal evolution and guide the clinical therapeutic strategies.
Methods: A total of 396 patients diagnosed with newly diagnosed MM (NDMM) between January 2014 and March 2020 were included in this study. EDTA-anticoagulated BM aspirate samples were collected at diagnosis for all 396 patients included in this study, and clonal PCs were enriched by CD138+ magnetic beads (Miltenyi Biotec, Paris, France). iFISH was then performed with probes specific for CAs as follows: 13q14, 17p13, 1q21, 14q32 (5’IGH, 3’IGH), t(4;14)(p16;q32), t(11;14)(q13;q32), and t(14;16)(q32;q23), and a total of 200 interphase nuclei were analyzed.
Results: Persistent CAs after induction therapy were detected in about half of the patients (118/269, 43%), and patients with undetectable CAs showed significantly improved survival compared with those with genetically detectable MRD (median progression-free survival [mPFS]: 59.7 months vs. 35.7 months, P < 0.001; median overall survival [mOS]: 97.1 months vs. 68.8 months, p = 0.011). Additionally, different patterns of therapy-induced clonal evolution were observed by comparing the clonal structure of residual PCs with paired baseline samples. Patients who maintained at high-risk during follow-up had the worst survival (mPFS: 23.0 months; mOS:40.4 months), while those returned to lower risk or had iFISH- at both time points had the best survival (mPFS: 59.7 months; mOS: 97.2 months).
Conclusions: These foundings highlighted the prognostic value of genetic testing in residual tumor cells after induction therapy, which may provide a deep understanding of clonal evolution and guide the clinical therapeutic strategies.
