Myeloma Novel Drug Targets and agents
Poster Session 2
P-193: Novel antigens LMA and KMA are expressed on malignant bone marrow plasma cells from patients at all stages of multiple myeloma and in other plasma cell dyscrasias
Thursday, September 28, 2023
12:30 PM - 1:30 PM EEST

Rosanne Dunn, PhD (she/her/hers)
Director and Chief Scientific Officer
Haemalogix Ltd
Woolloomooloo, New South Wales, Australia
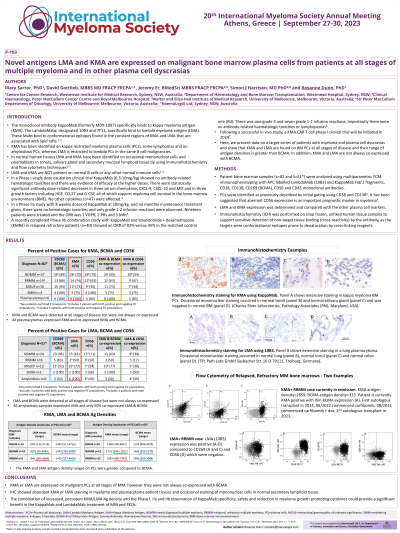
Introduction: As confirmed by flow cytometry and immunohistochemistry, lambda myeloma antigen (LMA) and kappa myeloma antigen (KMA) are found on the surface of malignant plasma cells (PCs) in multiple myeloma (MM) and other plasma cell dyscrasia (PCD) patient bone marrow (BM) samples, on human myeloma cell lines, on occasional mononuclear cells in healthy tonsillar tissue and secondary mucosal lymphoid tissue; they are not present on normal B cells in BM. KappaMab (formerly MDX-1097) clinical trials have confirmed that normal leukocytes are not depleted by the antibody with no on-target off-tumour effects1-3, and preclinical KMA.CAR T cell data has confirmed the antibody specificity4. KappaMab (targeting KMA) and LambdaMab (targeting LMA) bind to unique conformational epitopes in the constant regions of these sphingomyelin-associated light chains. This updated analysis confirms increased antigen (Ag) density of LMA and KMA on PCs versus decreased or stable Ag density of BCMA in disease progression from MGUS to relapsed, refractory MM (RRMM). Also, KMA is consistently found on BM PCs from plasmacytoma patients and LMA is consistently found on BM PCs from AL-amyloidosis (AL) patients.
Methods: Patient BM samples (κ=66 and λ=41) were analysed using multiparametric FCM immunophenotyping with APC labelled LambdaMab (10B3) and KappaMab Fab’2 fragments, CD38, CD138, CD269 (BCMA), CD319 (SLAM F7), CD56 and CD45 monoclonal antibodies. PCs were identified as previously described5 by initial gating using CD38 and CD138. LMA and KMA expression was compared with the other cell markers. Ag density was calculated using QuantiBrite beads in PE.
Results: In MGUS and SMM samples, 79% (11 of 14) of λ-type expressed LMA and 63% (10 of 16) of κ-type expressed KMA. KMA was expressed in 75% (12 of 16) of ĸRRMM samples and LMA was expressed in 50% (2 of 4) of λRRMM samples. Co-expression of KMA and BCMA occurred in 62% (18 of 29) of newly diagnosed (ND)MM and 63% (10 of 16) of RRMM samples. Co-expression of LMA and BCMA occurred in 59% (10 of 17) of NDMM and 50% (2 of 4) of RRMM samples. Both KMA and LMA Ag densities were higher than BCMA in the RRMM population. In the plasmacytoma BM PCs (n=5) KMA was expressed in 100% and KMA and BCMA were co-expressed in 100%. In the LMA+ AL samples (n=6), LMA was expressed in 100% and LMA and BCMA were co-expressed in 50%.
Conclusions: KMA or LMA are expressed on PCs at all stages of MM, and all plasmacytomas and AL patient samples. The increased Ag densities of both KMA and LMA compared to BCMA on RRMM BM PCs indicates antigen persistence on a treatment resistant clone. The combination of increased and persistent antigen density and the specificity of these therapeutic antibodies could provide a significant benefit in the treatment of myeloma and PCDs.
1. Spencer. BCJ. 2019;9:58; 2. Dunn. Haematologica. 2013; 98(s1):776; 3. Kalff. Blood. 2019;134(s1):3144; 4. Li, J. Cancer Res. 2023; 83 (7Supp): 4074; Sartor M. Blood. 2021;138(Supp 1):1595
Methods: Patient BM samples (κ=66 and λ=41) were analysed using multiparametric FCM immunophenotyping with APC labelled LambdaMab (10B3) and KappaMab Fab’2 fragments, CD38, CD138, CD269 (BCMA), CD319 (SLAM F7), CD56 and CD45 monoclonal antibodies. PCs were identified as previously described5 by initial gating using CD38 and CD138. LMA and KMA expression was compared with the other cell markers. Ag density was calculated using QuantiBrite beads in PE.
Results: In MGUS and SMM samples, 79% (11 of 14) of λ-type expressed LMA and 63% (10 of 16) of κ-type expressed KMA. KMA was expressed in 75% (12 of 16) of ĸRRMM samples and LMA was expressed in 50% (2 of 4) of λRRMM samples. Co-expression of KMA and BCMA occurred in 62% (18 of 29) of newly diagnosed (ND)MM and 63% (10 of 16) of RRMM samples. Co-expression of LMA and BCMA occurred in 59% (10 of 17) of NDMM and 50% (2 of 4) of RRMM samples. Both KMA and LMA Ag densities were higher than BCMA in the RRMM population. In the plasmacytoma BM PCs (n=5) KMA was expressed in 100% and KMA and BCMA were co-expressed in 100%. In the LMA+ AL samples (n=6), LMA was expressed in 100% and LMA and BCMA were co-expressed in 50%.
Conclusions: KMA or LMA are expressed on PCs at all stages of MM, and all plasmacytomas and AL patient samples. The increased Ag densities of both KMA and LMA compared to BCMA on RRMM BM PCs indicates antigen persistence on a treatment resistant clone. The combination of increased and persistent antigen density and the specificity of these therapeutic antibodies could provide a significant benefit in the treatment of myeloma and PCDs.
1. Spencer. BCJ. 2019;9:58; 2. Dunn. Haematologica. 2013; 98(s1):776; 3. Kalff. Blood. 2019;134(s1):3144; 4. Li, J. Cancer Res. 2023; 83 (7Supp): 4074; Sartor M. Blood. 2021;138(Supp 1):1595
